Introduction: Pivotal CAR-T registrational trials for the six CAR-T therapies currently FDA approved have excluded those with heart failure (HF), defined either by low ejection fraction (EF) or clinical symptoms of HF (NYHA functional classification). This has created a real-world dilemma for clinicians desiring to treat this patient population. There is concern that those with HF may not tolerate the physiologic and hemodynamic stressors associated with cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) and thus have increased risk of severe morbidity or death. The emerging concept of permissive cardiotoxicity seeks to allow for an acceptable level of cardiac toxicity to allow for treatment with highly efficacious but potentially cardiac toxic treatments like CAR-T therapy. To that end, in this study, we provide our real-world, single-institution experience with CAR-T therapy in patients with medically optimized HF with reduced EF (HFrEF) prior to date of apheresis.
Methods: This was a retrospective review of 168 (100 B-cell lymphoma and 68 multiple myeloma) CAR-T therapy patients, with 20 (12%) identified with HFrEF of ≤50% prior to apheresis by two-dimensional transthoracic echocardiogram as part of the standard CAR-T screening process and without clinical symptoms of HF at time of apheresis. 19/20 patients received standard of care CAR-T products. This study was conducted following IRB approval. Statistical analysis was done using R version 4.2.3. Kaplan-Meier curves were used to calculate progression free survival(PFS) and overall survival(OS).
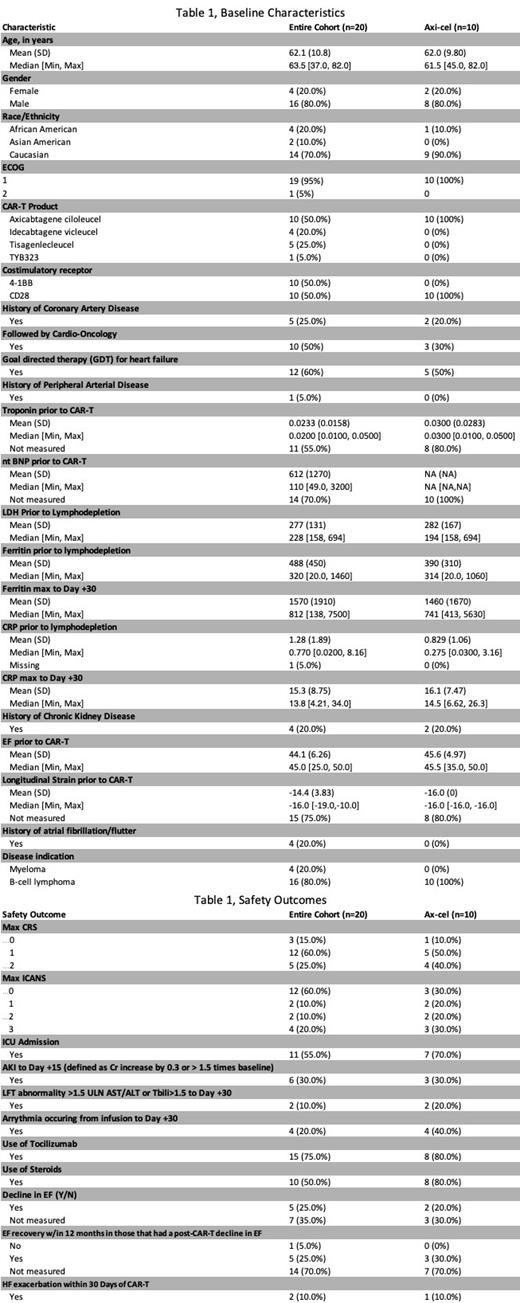
Results: Twenty patients with HFrEF prior to CAR-T infusion were identified with median EF of 44.1% (range 25-50%) (see Table 1, Baseline Characteristics). The median age was 63.5 (37-82) years-old, predominately male gender (80%), Caucasian (70%), with ECOG of 1 (95%), and 10 patients (50%) received axicabtagene ciloleucel (axi-cel). Most patients received CAR-T for B-cell lymphoma (80%). Cardiac history included 5 (25%) with previous CAD, 1 (5%) with previous PAD, and 4 (20%) with history of atrial fibrillation/flutter. Cardio-Oncology was consulted prior to CAR-T therapy in 10 (50%) and 12 (60%) were optimized on goal-directed therapy (GDT). CKD was pre-existing in 4 (20%) patients. Where measured, all patients had a pre-CAR-T troponin within normal institutional limits (<0.05ng/mL). NT-BNP at baseline was only measured in 6 (30%) patients with a median value of 110pg/mL (49-3200, upper limit of normal 125pg/mL). Longitudinal strain prior to CAR-T was measured in 5 (25%) patients with median value of -16% (-19 to -10%, <-17% normal).
There were no cases of grade ≥3 CRS with 3 (15%) grade 1 and 12 (60%) grade 2 CRS (see Table 1, Safety Outcomes). There were 4 (20%) with grade 3 ICANS, although most patients 12 (60%) did not experience ICANS. Compared to ZUMA-1, the 10 patients receiving axi-cel did not have significantly higher rates of CRS or ICANS. Tocilizumab was administered in 15 (75%) of patients and steroids given in 10 (50%) of patients. Following CAR-T infusion, 11 (55%) of patients required ICU admission, no patients required vasopressors, 6 (30%) experienced an AKI, 2 (10%) had LFT abnormalities, 4 (20%) had an arrhythmia, and 5 (25%) had further decline in EF with recovery occurring in most cases where measured. Only 2 (10%) of patients had an HF exacerbation within 30 days of CAR-T infusion. PFS and OS were 11.3 and 26 months, respectively.
Conclusion: Given that all registrational CAR-T trials excluded HF, notable reservation exists among clinicians in the use of CAR-T therapy in patients with a history of HF and particularly HFrEF. In our institutional experience of 20 patients with good performance status and medically optimized on GDT, we demonstrate that CAR-T therapy can be given safely with rates of CRS and ICANS similar to that expected in the non-HF population. Additionally, while ICU utilization was high, the incidence of arrhythmias, renal or hepatic toxicity as indicators of end organ dysfunction, non-recovering EF decline, and heart failure exacerbation following CAR-T infusion were quite low. It is thus our practice to consider CAR-T therapy on a case by case basis in those with a history of HFrEF. The key to success being maximal cardiac optimization achieved through early engagement of our Cardio-Oncology colleagues based upon the concept of permissive cardiotoxicity.
Disclosures
Hoffmann:BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Honoraria. McGuirk:Astellas Pharma: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding; Pluristem Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal